About TBMC
The rapid progress, production, and global distribution of mRNA vaccines have played a pivotal role in combating the effects of COVID-19 worldwide. In the early stages of vaccine rollout, the supply of mRNA vaccines was primarily directed to the countries engaged in their development and production, leading to a temporary shortage of vaccines in Taiwan.
Consequently, a country‘s ability to quickly manufacture advanced biological pharmaceuticals through Contract Development and Manufacturing Organization (CDMO) services is closely linked to protecting the health and welfare of its citizens.
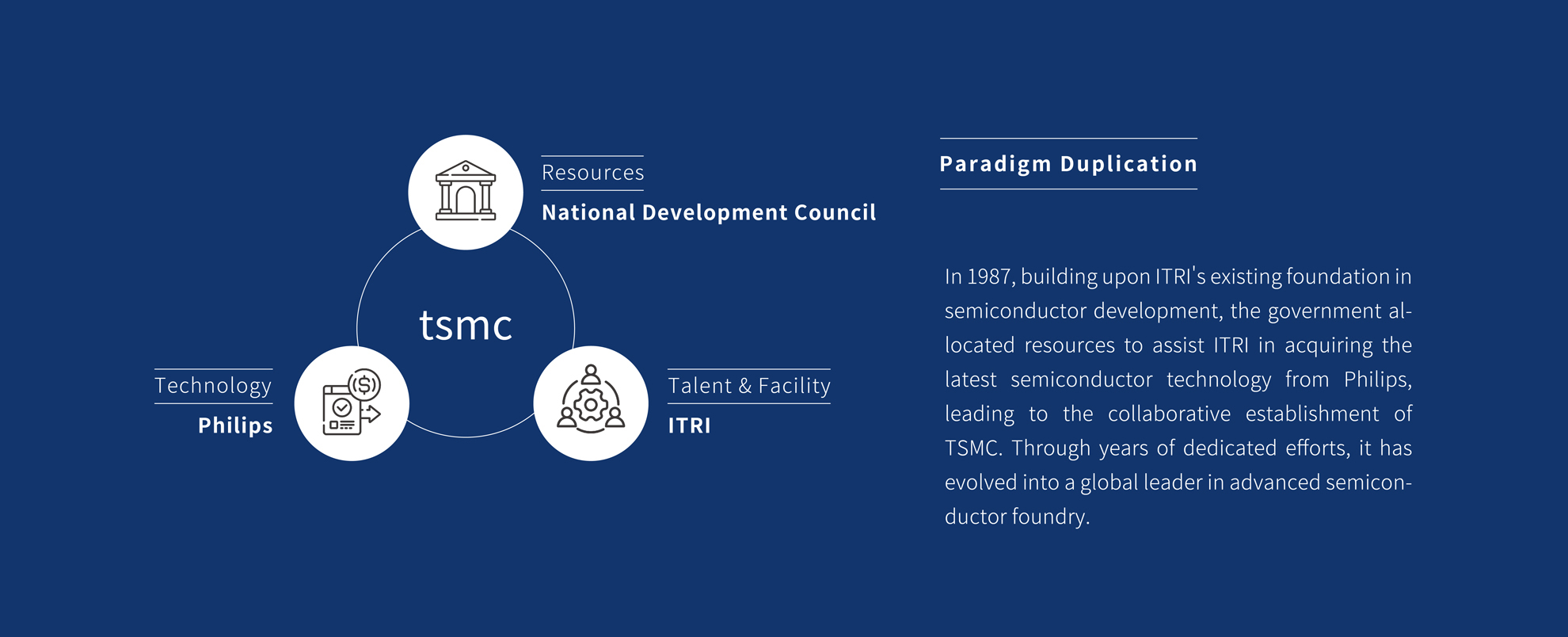
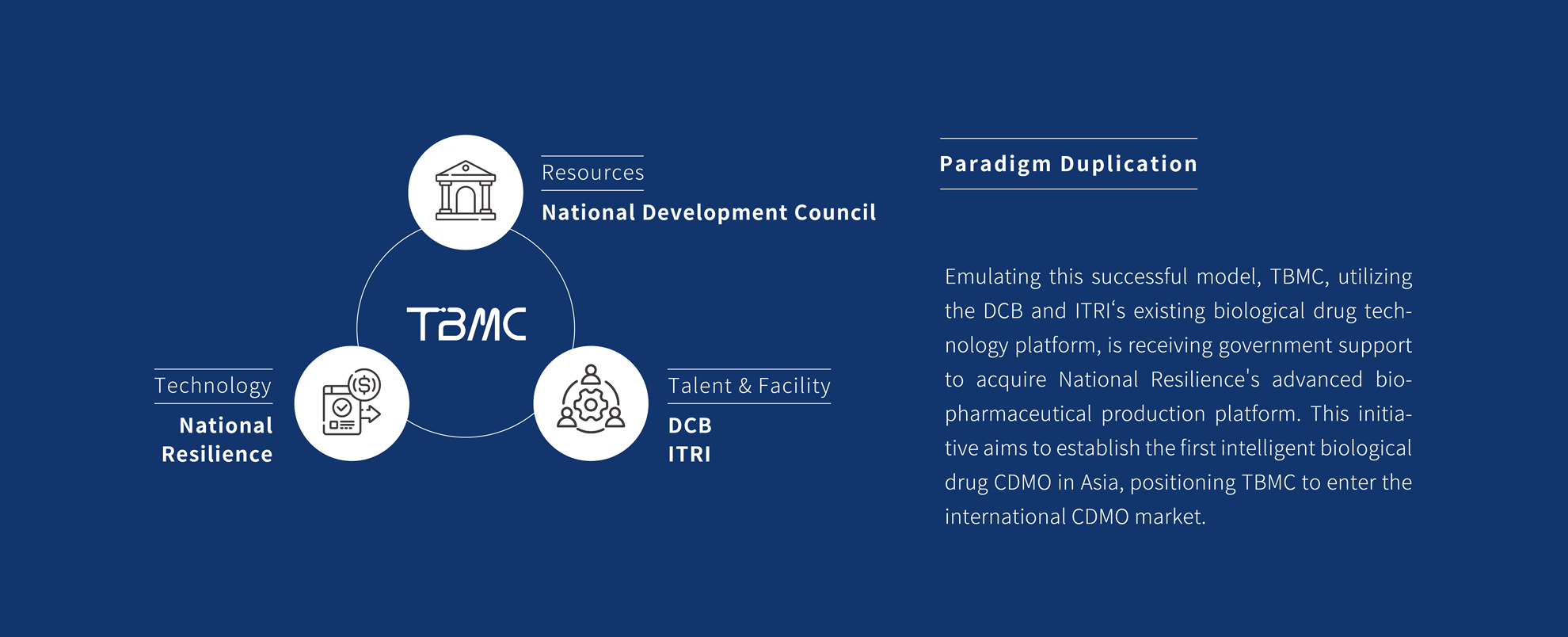
Taiwan Bio-Manufacturing Corporation (TBMC), established in May 2023, specializes in cutting-edge biotechnology, including Gene Therapy, cell therapies, and protein technologies. Born from collaborations with the Department Center for Biotechnology (DCB) and Industrial Technology Research Institute (ITRI), TBMC is rapidly advancing in biopharmaceutical manufacturing.
With state-of-the-art technologies like Raman spectroscopy and automated cell culturing system, TBMC aims to become Asia's first Pharma 4.0 CDMO, delivering high-quality biologics medicines.
Previous
Next
Vision
At TBMC, our vision is to become a world-class bio-manufacturing CRDMO, recognized for innovation, reliability, agility, and efficiency. We proudly operate by our guiding principle of unwavering competitiveness, consistency and commitment. We are dedicated to building an inspired team by hiring only the best talent and fostering a culture of continuous motivation and excellence.
Mission
Our mission is to manufacture advanced biopharmaceuticals using cutting-edge technologies that deliver maximum efficiency, speed, yield, and production success. We are committed to bringing the highest standards of biomanufacturing to our partners and the patients they serve.
Previous
Next
We believe true success lies in the disciplined
execution of six key pillars:
Customer satisfaction and trust
Technical Prowess
Global Reach
Engrained End-to-End Quality
Strong Leadership and Engaged Workforce
Culture of Accountability and Execution
These principles strengthen our competitiveness, elevate us to world-class standards, and enable us to lead and create value to the global biomanufacturing industry.
Previous
Next
Milestone
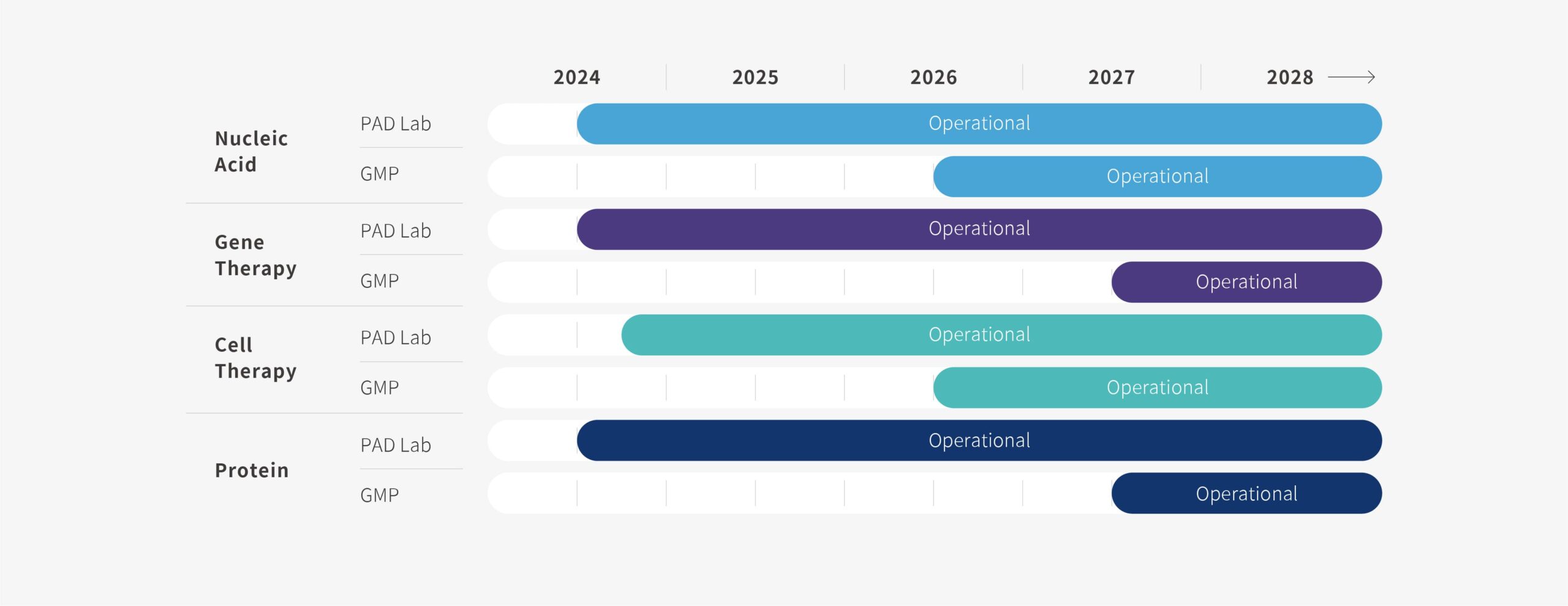
Process Development Lab
Operational since 2024 Q2
Taipei Bioinnovation Park
1,820 m2

Cell Therapy Process Development Lab
Operational since 2024 Q4
GMP Plant
Operational Ready by 2026 Q2
Hsinchu Biomedical Science Park
11,900 m2

Process Development Lab
Operational since 2024 Q2
Taipei Bioinnovation Park
1,820 m2

Cell Therapy Process Development Lab
Operational since 2024 Q4
GMP Plant
Operational Ready by 2026 Q2
Hsinchu Biomedical Science Park
11,900 m2

Team

Patrick Yang
Ph. D.
Chairman/ Director of the Board

David Chang
Ph. D.
CEO

Wei-Kuang Chi
Ph. D.
CTO

Jennifer Tseng
General Counsel

Michel Chu
Chief Strategy Advisor

Chien-Cheng Tai
Ph. D.
Director of the Board
Representative of the National Development Fund Management Committee

Yi-Hui Lin
Director of the Board
Representative of the National Development Fund Management Committee

Karen Yu
Director of the Board
Representative of the Industrial Technology Investment Corporation

Yao-Hwa Co., Ltd Management Commission
Vice Chairman/ Director of the Board

Rahul Singhvi, SC.D.
Director of the Board
Representative of RTW23, LLC

Audrey Tseng
Supervisor



